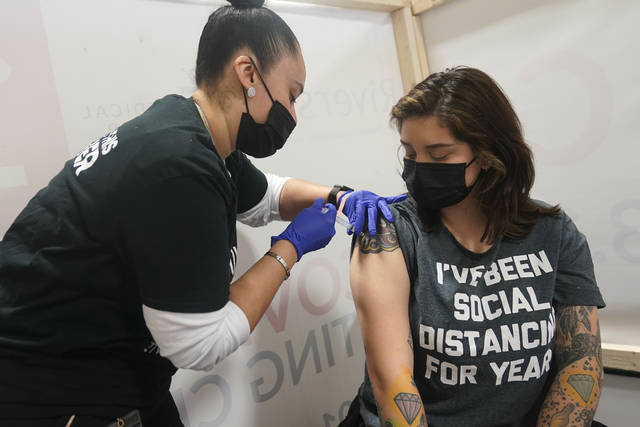
WASHINGTON — A U.S. advisory panel has endorsed the new one-dose covid-19 vaccine from Johnson & Johnson as a third option to bolster the national effort against the coronavirus pandemic.
Advisers to the Centers for Disease Control and Prevention voted overwhelmingly to recommend the vaccine for adults 18 years old and up. The ruling followed emergency clearance of the vaccine by U.S. regulators a day earlier.
Members of the group emphasized that all three vaccines now available in the U.S. are highly protective against the worst effects of the virus, including hospitalization and death.
J&J plans to ship several million vaccine doses to states in the coming week, delivering a total of 20 million shots by the end of March. Health officials are eager to have an easier-to-use vaccine against covid-19, which has killed more than 511,000 Americans and continues to mutate in troubling ways.
CDC recommendations are not binding on state governments or doctors, but are widely heeded by the medical community. The same CDC panel previously recommended use of the two vaccines from Pfizer and Moderna authorized in December.