WASHINGTON — U.S. government officials are launching a new study testing three drugs to tamp down an overactive response by the immune system that can cause severe illness or death in people with covid-19.
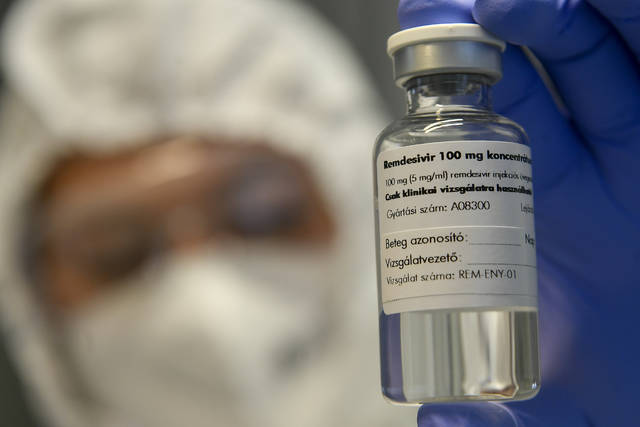
The U.S. National Institutes of Health says the study will enroll 2,100 hospitalized adults with moderate to severe covid-19 in the United States and Latin America. All will get the antiviral drug remdesivir plus one of the three “immune-modulating drugs” or a placebo.
The drugs are Bristol Myers Squibb’s Orencia and Johnson & Johnson’s Remicade, which are sold now for rheumatoid arthritis and an experimental drug from AbbVie called cenicriviroc. The drugs work in different ways to inhibit “cytokine storm,” an overproduction of chemicals the body makes to fight infections that can damage lungs, kidneys, the heart and other organs.
“These are all different ways of slowing down an overactive immune system,” said NIH director Dr. Francis Collins.
The new study is the fifth and final one in a series of experiments designed by a private-public partnership that includes dozens of drug companies, nonprofit groups and various U.S. government departments. Other therapies being tested include antibody drugs, anti-inflammatory medicines and plasma from covid-19 survivors.